FDA’s Enforcement Actions Raise Broad Concerns Regarding Indonesian Shrimp
August 20, 2025 | 8 min to read
This year, the FDA has intensified its scrutiny of Indonesian shrimp, particularly targeting PT. Bahari Makmur Sejati for inclusion on Import Alert 99-51 due to Cs-137 contamination. The agency reported a notable increase in entry line refusals, citing issues such as banned veterinary drug residues, undeclared sulfites, and contamination with salmonella. Such stringent measures reflect ongoing concerns about food safety and adherence to regulations within the shrimp industry.

This year, the U.S. Food and Drug Administration (“FDA”) appears to have cracked down on shipments of Indonesian shrimp for a variety of disturbing reasons.
Recently, the FDA added Indonesia’s PT. Bahari Makmur Sejati (“BMS”) to Import Alert 99-51, Detention without Physical Examination of Human Food Products that Appear to Have Been Prepared, Packed or Held Under Unsanitary Conditions Resulting in Chemical Contamination, for Cs-137 in “All Shrimp Products” on August 14, 2025.
Import Alert 99-51 is rarely used and, at present, does not list any entity other than Indonesia’s BMS. Explaining that the agency oversees the safety of the U.S. food supply by monitoring for contaminants in food, the FDA’s Import Alert describes Cs-137 as follows:
[R]adionuclides such as Cesium-137 (Cs-137), a radioisotope of cesium, can be present in many places around the world as a result of contamination produced high in the atmosphere during nuclear testing. Cs-137 is created via nuclear reactions, such as occurring in nuclear power plants and is also commonly used in medical and other industrial applications. Elevated amounts of Cs-137 can be present at locations where contamination settled from accidents such as Chernobyl in 1986 and Fukushima in 2011.
The Import Alert describes the risks to health created by exposure to low doses of Cs-137 as:
Cs-137 emits beta particles and gamma radiation that are associated with adverse health effects. Potential for health concerns following Cs-137 exposure depends on the dose and the duration of exposure. High doses lead to acute radiation syndrome. However, exposure to low doses spread out over a period of time may not cause immediate apparent adverse effects but may still be harmful. The primary health effect of concern following longer term, repeated low dose exposure (e.g., through consumption of contaminated food or water over time) is cancer, resulting from damage to DNA within living cells of the body.
There has been no explanation as to why Cs-137 would be present in Indonesian shrimp exported from BMS to the United States. In the agency’s public comments about its response to the release of radioactive wastewater from the Fukushima Daiichi nuclear power station in Japan, the FDA stated that although “Cs-137 has a half-life of thirty years . . . [it] is readily excreted and does not accumulate in seafood.”
The problems that the FDA has found with Indonesian shrimp this year extend well beyond radioactive isotopes. As shown in the chart below, so far this year, the FDA has refused an extraordinary number of entry lines of Indonesian shrimp.
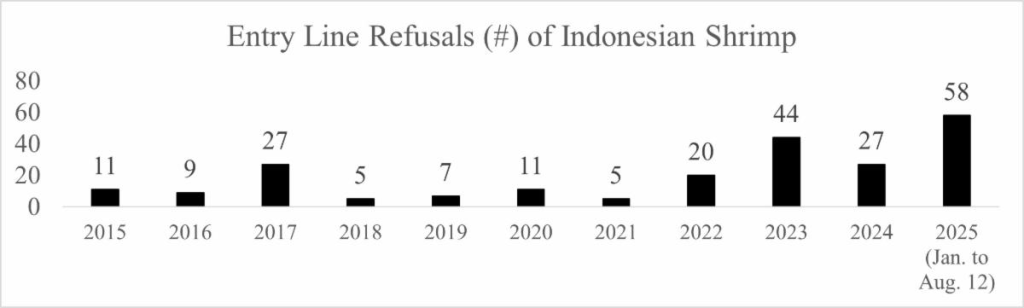
With several more months of the year left to go, the FDA has refused more entry lines of Indonesian shrimp this year than any year since 2011 when the agency refused 80.
These refusals have been for a variety of different reasons. As described in more detail below, for the first time in many years, the FDA has once again been finding banned veterinary drug residues in Indonesian shrimp. Further, the federal agency has also rejected a number of shipments for undeclared sulfites and has continued actions taken last year to counter economic fraud through short-weighting and miscounts.
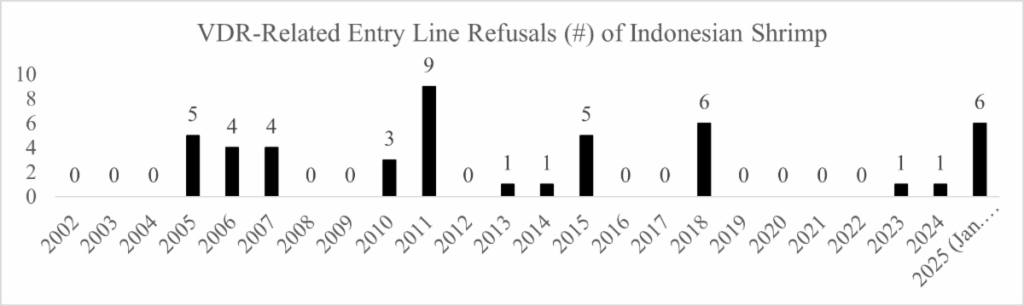
Between 2002 and 2024, the FDA reported just forty (40) total refusals of shrimp entry lines from Indonesia for reasons related to veterinary drug residues. Yet, in the first eight months of this year, the FDA has refused six entry lines of Indonesian shrimp exported by PT. Pabrik Lamongan BMI (5) and PT. Tamron Akuatik Produk Industri (1) for nitrofurans and/or veterinary drug residues. For its part, PT. Pabrik Lamongan BMI has been listed on the FDA’s Import Alert 16-129, Detention Without Physical Examination of Seafood Products Due to Nitrofurans, for its shrimp since June 12, 2025.
Another thirty-four (34) entry lines of Indonesian shrimp exported by Mega Marine Pride were refused this year for undeclared sulfites.
Shipments from Mega Marine Pride also comprised two of the seven entry line refusals of Indonesian shrimp for added bulk, joining shrimp exports from PT Bumi Pangan Utama (2) and PT. First Marine Seafoods (3). Moreover, another two entry lines – one from PT. Pabrik Lamongan BMI and one from Mega Marine Pride – were refused for a failure to bear accurate statements regarding weight, measures, or numerical counts. Relatedly, the FDA’s Import Alert 99-47, Detention Without Physical Examination of Human Food Products that Appear to Be Adulterated for Economic Gain, currently lists three Indonesian shrimp exporters: Mega Marine Pride (added Feb. 27, 2025); PT Bumi Pangan Utama (added May 16, 2025); and PT. First Marine Seafoods (added Aug. 1, 2025). Outside of these Indonesian companies, there is only one other shrimp exporter – India’s Asvini Fisheries Private Limited (added Aug. 6, 2025) – currently included in Import Alert 99-47.
Finally, nine entry lines of Indonesian shrimp, all from PT Mustika Minanusa Aurora, were refused this year for contamination with salmonella and for being deemed “filthy.” Another entry line of shrimp from PT. Pabrik Lamongan BMI was also refused this year for being filthy. Nevertheless, despite the findings of salmonella in shrimp shipped by PT Mustika Minanusa Aurora and PT. Pabrik Lamongan BMI, both of these Indonesian companies have been exempted from the protective measures taken by the FDA to address filth and salmonella in Indonesian shrimp through inclusion on the “Green List” of Import Alert 16-18, Detention Without Physical Examination of Raw Shrimp, since July 31, 2023.
About the Southern Shrimp Alliance
The Southern Shrimp Alliance (SSA) is an organization of shrimp fishermen, shrimp processors, and other members of the domestic industry in the eight warmwater shrimp producing states of Alabama, Florida, Georgia, Louisiana, Mississippi, North Carolina, South Carolina, and Texas.
